Reliable binding isotherms and affinities despite problematic exchange regimes in NMR spectra, using TREND NMR software
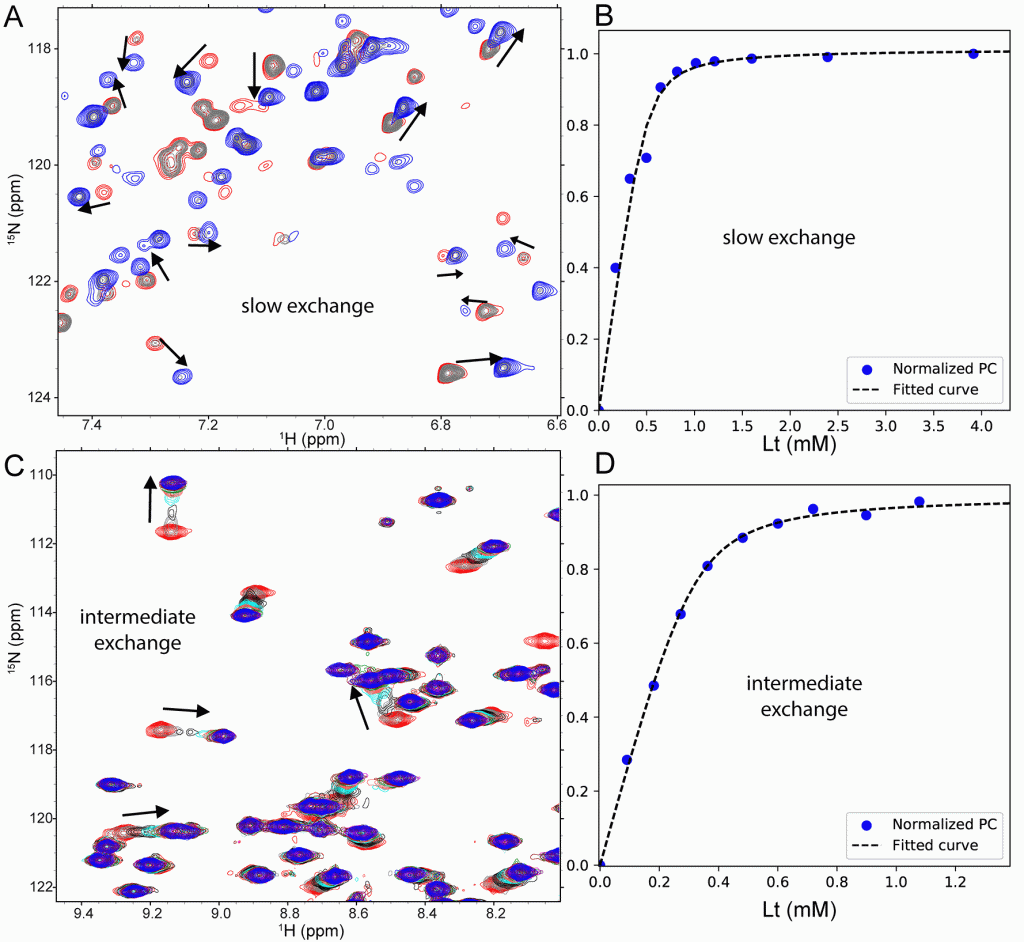
Panel (A) shows application to spectra with abrupt jumps of NMR peak position (slow exchange) upon titration of a 52 kDa enzyme with an inhibitor. Panel (B) shows the binding isotherm calculated automatically by TREND NMR and fitted to a KD and plotted with TRENDanalysis. Panel (C) shows a titration with a mixture of both linear peak shifts (fast exchange) and nonlinear shifts with peak broadening (intermediate exchange). A phosphopeptide was titrated into an FHA domain and monitored by 15N HSQC spectra across the range of concentrations in the titration. Panel (D) shows the binding isotherms automatically computed by TREND NMR using Pareto scaling to assist with the nonlinearity of peak shifts present
